 Home Home
 What the Perth Group has argued What the Perth Group has argued
 Papadopulos redox theory of cellular function papers Papadopulos redox theory of cellular function papers
 Papers and letters published in scientific journals Papers and letters published in scientific journals
 Monograph on mother-to-child transmission Monograph on mother-to-child transmission
 Papers published in Continuum magazine Papers published in Continuum magazine
 Papers published in the popular press Papers published in the popular press
 Papers/letters rejected by the scientific press Papers/letters rejected by the scientific press
 Presentations Presentations
 Interviews Interviews
 Selected email correspondence Selected email correspondence
 A virus like no other A virus like no other
 Oxidation, Montagnier and the Perth Group Oxidation, Montagnier and the Perth Group
 Montagnier Nobel Prize 2008 Montagnier Nobel Prize 2008
 The Parenzee Case The Parenzee Case
 The House of Numbers The House of Numbers
 Latest files Latest files
 National Libary of Australia Intervew 1993 National Libary of Australia Intervew 1993
 Others Others
 Africa/South Africa Africa/South Africa
 Questions and answers Questions and answers
 Response to the NIH "Evidence" that HIV causes AIDS Response to the NIH "Evidence" that HIV causes AIDS
 Translations of the Perth Group papers Translations of the Perth Group papers
 BMJ Online Debate BMJ Online Debate
 Links Links
 Contact Us Contact Us
 About the Perth Group About the Perth Group
 Perth Group at Virusmyth Perth Group at Virusmyth
 The Perth Group on YouTube The Perth Group on YouTube
|
“Anti-HIV
antibodies”, ARVs and informed consent
Note: This letter was rejected by the Medical Journal of Australia on 12th
August 2003.
The response was “Many thanks for your recent contribution to the Journal. Your
manuscript was discussed at the Editorial Committee Meeting and unfortunately
the outcome was that we would not proceed with peer review or publication. As
you are probably aware, the competition for space in the journal is now
particularly fierce and we have to, by necessity, reject many deserving
articles. We are sorry to disappoint you and hope you are successful in securing
publication elsewhere. With kind regards, Dr. Ann T Gregory, Deputy Editor”
Paper at
www.mja.com.au/public/issues/178_11_020603/osu10738_fm.html
================================================================
In a recent study1 published in the Journal prisoners were offered
antiretroviral drugs on the basis that a positive antibody test is proof for
transmission of an AIDS causing retrovirus HIV. Although this represents the
majority view, the evidence in its favour is not without problems.2-5 Antibodies
are polyspecific and are apt to yield confounding cross-reactivities.6, 7 These
are particularly likely in AIDS patients for several reasons including their
typical hypergammaglobulinaemia and the fact that 90% of AIDS diagnoses involve
fungal or mycobacterial agents8 which induce antibodies reactive with the
antigens employed in the antibody test kits.9-12.
Proof that the antigens in the test kits react exclusively with antibodies
directed against a retrovirus HIV can be obtained only by comparing the
reactions with HIV itself. That is, HIV isolation is the only scientifically
valid gold standard for determining the specificity of a test claimed to
diagnose HIV infection. Yet to date there are no such data, a caveat repeatedly
included in the packet insert of one test manufacturer: “At present, there is no
recognized standard for establishing the presence or absence of antibodies to
HIV-1 and HIV-2 in human blood”.13 Instead of using the correct gold standard
HIV experts and biotechnology companies determine specificity for HIV using AIDS
as a gold standard. Not only is this not the professed outcome of the test,14 if
AIDS is used as a gold standard then by definition, all seropositive, AIDS-free
individuals including gay men, drugs users, haemophiliacs, Africans and those
not in a defined risk group, healthy or otherwise, must be false positives.
Given the majority of seropositive individuals fall into these categories, no
such individuals can be infected with HIV, the positive predictive value of the
tests is insignificant and “the global burden of HIV” unsubstantiated.
Failure to use an HIV isolation gold standard has also resulted in the bizarre
situation where globally there are at least ten different sets of criteria for
defining a positive, “confirmatory”, HIV Western blot (WB) (Figure 1). The
consequences are, for example, an individual “confirmed” infected in New York
City on the CDC criteria would not be “confirmed” infected in Sydney, Australia.
Or an Australian WB positive with p41, p32, p24 and p18 bands would not be
“confirmed” infected in Africa. Or an African WB positive with a p41 and p120
band would not be positive in Australia, parts of the USA or Europe. Or, as
reported in the study by Kashala,11 up to 83% of African leprosy patients and
64% of their contacts have a positive WB pattern not considered positive by the
World Health Organisation but considered positive by the most “stringent”
criteria of the Australian National Serology Reference Laboratory. This
confusion is confirmed in laboratory manuals, one of which advises, “Specific
guidelines for interpretation may differ depending on the local policies,
GENELABS recommends following the accepted policy to be in accordance with local
regulations”. It then adds further to the confusion by appending yet another set
of criteria for a positive Western blot.15 It seems remarkable that “local
policies” and “local regulations”, rather than the biological properties of a
virus, determine WB band patterns regarded as specific proof of infection and
that such extensive variation is regarded consistent with “extraordinarily
accurate” antibody tests.16
Within the risk groups a positive antibody test, whatever its genesis,
correlates with the presence or development of the clinical AID syndrome. This
relationship is not under contention. What the minority view disputes is the
claim that data based on an AIDS gold standard prove the tests specific for a
retroviral infection. In no way does this view preclude the tests possessing a
high degree of clinical relevance. Physicians are familiar with several
non-specific laboratory tests of diagnostic and prognostic utility. For example,
an elevated erythrocyte sedimentation rate (ESR) is caused by "the dielectric
effect of proteins in the surrounding plasma", especially "fibrinogen,
immunoglobulins, and other acute-phase reaction proteins", and their increased
levels in some disease states.17 Values exceeding 100 mm/hr have a 90% positive
predictive value for serious underlying pathology including infection, collagen
vascular disease or metastatic tumours.18 Thus, like a positive antibody test,
an elevated ESR also predicts a number of unrelated diseases including AIDS
indicator diseases19 and encompasses an increased probability of dying within
the next several years.
These matters have significant implications now that the November 1992
Australian High Court ruling on Rogers-v-Whittaker obliges physicians to provide
patients with “all the relevant information to choose between undergoing and not
undergoing the treatment”.20 The same principle is echoed in the AMA Code of
Ethics, “Make sure that all research participants or their agents are fully
informed”,21 and in the Helsinki Declaration, “In any medical study, every
patient – including those of a control group, if any –should be assured of the
best proven diagnostic and therapeutic method”.22 Prisoners are no exception to
the need for informed consent with treatments based on the interpretation of a
laboratory test. In fact they present an extra ethical dimension because they
are held in captivity and, rightly or wrongly, may feel coerced into accepting
treatments which are potentially toxic. Neither is the issue of providing full,
relevant information a legal obligation only for prisoners. There may be other
individuals reluctant to question their physicians’ good intentions or who feel
unable to deal with a doctor who lacks awareness of other scientific opinion.
This places patients at risk of passively accepting treatments they might
otherwise defer or reject. Physicians and researchers offering treatments should
acquaint their patients with the scientific basis of the tests they use
including scientific opinion at variance with their own.
REFERENCES
1. O'Sullivan BG, Levy MH, Dolan KA, Post JJ, Barton SG, Dwyer DE, et al.
Hepatitis C transmission and HIV post-exposure prophylaxis after needle- and
syringe-sharing in Australian prisons. Med J Aust 2003;178:546-9.
2. Papadopulos-Eleopulos E, Turner VF, Papadimitriou JM. Is a positive Western
blot proof of HIV infection? Bio/Technology 1993;11:696-707.
3. Papadopulos-Eleopulos E, Turner VF, Papadimitriou JM, Causer D. HIV
antibodies: Further questions and a plea for clarification. Curr Med Res Opinion
1997;13:627-634.
4. Papadopulos-Eleopulos E, Turner VF, Papadimitriou JM, Causer D, Page B. HIV
antibody tests and viral load - more unanswered questions and a further plea for
clarification. Curr Med Res Opinion 1998;14:185-186.
5. Papadopulos-Eleopulos E, Turner VF, Papadimitriou JM, Alfonso H, Page BA,
Causer D. A critical analysis of the evidence for the existence of HIV. Online
BMJ 2003.
http://bmj.com/cgi/eletters/326/7387/495#31507
6. Nossal GJV. Antibodies and
Immunity. Harmondsworth, UK: Penguin Books Ltd; 1971.
7. Ternynck T, Avrameas S. Murine natural monoclonal antibodies: a study of
their polyspecificities and their affinities. Immunol Rev 1986;94:99-112.
8. Hu DJ, Fleming PL, Castro KG, Jones JL, Bush TJ, Hanson D, et al. How
important is race/ethnicity as an indicator of risk for specific AIDS-defining
conditions? J Acquir Immune Defic Syndr Hum Retrovirol 1995;10:374-380.
9. Muller WEG, Bachmann M, Weiler BE, Schroder HC, Uhlenbruck GU, Shinoda T, et
al. Antibodies against defined carbohydrate structures of Candida albicans
protect H9 cells against infection with human immunodeficiency virus-1 in vitro.
J Acquir Immun Defic Syndr 1991;4:694-703.
10. Matthews R, Smith D, Midgley J, Burnie J, Clark I, Connolly M, et al.
Candida and AIDS: Evidence for protective antibody. Lancet 1988;ii:263-266.
11. Kashala O, Marlink R, Ilunga M, Diese M, Gormus B, Xu K, et al. Infection
with human immunodeficiency virus type 1 (HIV-1) and human T cell lymphotropic
viruses among leprosy patients and contacts: correlation between HIV-1
cross-reactivity and antibodies to lipoarabinomannan. J Infect Dis
1994;169:296-304.
12. Tessema TA, Bjune G, Hamasur B, Svenson S, Syre H, Bjorvatn B. Circulating
antibodies to lipoarabinomannan in relation to sputum microscopy, clinical
features and urinary anti-lipoarabinomannan detection in pulmonary tuberculosis.
Scand J Infect Dis 2002;34(2):97-103.
13. Packet Insert Axsym system (HIV-1/HIV-2). Abbott LaboratoriesDiagnostics
Division. 100 Abbott Park Rd. Abbott Park. Illinois: United States of America.
1988, 1998.
14. Griner PF, Mayewski RJ, Mushlin AI. Selection and interpretation of
diagnostic tests and procedures. Ann Int Med 1981;94:559-563.
15. Genelabs Diagnostics Pty Ltd HIV BLOT 2.2 Instruction Manual. Singapore;
1999.
16. National Institute of Allergy and Infectious Diseases. Focus on the HIV-AIDS
Connection. 2001.
www.niaid.nih.gov/newsroom/focuson/hiv00/default.htm
17. Wintrobe WM, Richard Lee G, Boggs
DR, Bithell TC, Foerster J, Athens JW, et al. Clinical Hematology. 8th ed.
Philadelphia: Lea & Febiger; 1981.
18. Brigden M. The erythrocyte sedimentation rate. Still a helpful test when
used judiciously. Postgrad Med 1998;103:257-62, 272-4.
19. Papadopulos-Eleopulos E, Turner VF, Papadimitriou JM, Alfonso H, Page BAP,
Causer D, et al. High rates of HIV seropositivity in Africa-alternative
explanation. Int J STD AIDS 2003;14:426-427.
20. Rogers v. Whitaker (1992) 175 CLR 479 F.C. 92/045. The High Court of
Australia; 1992.
www.austlii.edu.au/cgi-bin/disp.pl/au/cases/cth/high%5fct/175clr479.html?query=%22roger%22+and+%22whitaker%22
21. Australian Medical Association
Code of Ethics 2003.
www.ama.com.au/web.nsf/doc/WEEN-5M4VJV
22. World Medical Association. The
Declaration of Helsinki; 2000.
www.wma.net/e/ethicsunit/pdf/chapter_4_decl_of_helsinki.pdf
Global criteria defining a positive HIV Western blot
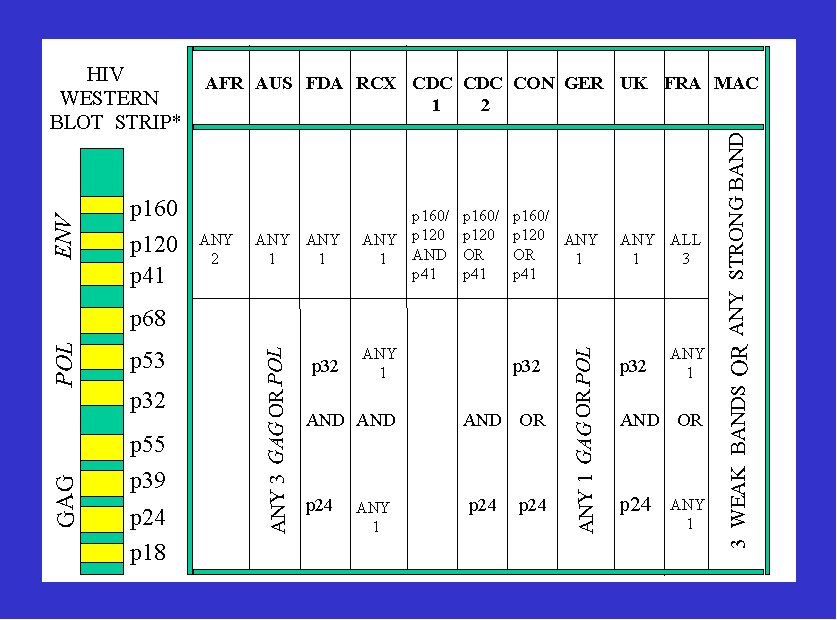
AFR=Africa1 AUS=Australia2 FDA=US Food and Drug Administration3 RCX=US Red
Cross3 CDC=US Centers for Disease Control3 CON=US Consortium for Retrovirus
Serology Standardization3 GER=Germany UK=United Kingdom FRA=France MACS= US
Multicenter AIDS Cohort Study 1987-1992. * Bands not in electrophoretic order
NOTES:
I. “The Association of Public Health Laboratories now recommends that patients
who have minimal positive results on the WB, eg p24 and gp160 only, or gp41 and
gp160 only, be told that these patterns have been seen in persons who are not
infected with HIV and that follow-up testing is required to determine actual
infective status”.4
II. In February 1993 the US Food and Drug Administration relaxed their criteria
in order to “reduce the number of HIV-1 seroindeterminate Western blot
interpretations”, that is, to increase the number of HIV positive individuals.5
1. WHO. (1990). Acquired Immunodeficiency Syndrome (AIDS). Proposed criteria for
interpreting results from Western blot assays for HIV-1, HIV-2 and
HTLV-I/HTLV-II. Weekly Epidemiological Record 65:281-298.
2. Healy DS, Maskill WJ, Howard TS, et al. (1992). HIV-1 Western blot:
development and assessment of testing to resolve indeterminate reactivity. AIDS
6:629-633.
3. Lundberg GD. (1988). Serological Diagnosis of Human Immunodeficiency Virus
Infection by Western blot Testing. Journal of the American Medical Association
260:674-679. (Data presented in this paper reveal that when the FDA criteria are
used to interpret the HIV Western blot less than 50% of US AIDS patients are HIV
positive whereas 10% of persons not at risk of AIDS are also positive).
4. Mylonakis E, Paliou M, Greenbough TC, Flaningan TP, Letvin NL, Rich JD.
Report of a false-positive HIV test result and the potential use of additional
tests in establishing HIV serostatus. Archives of Internal Medicine
2000;160:2386-8.
5. Keinman S, Busch MP, Hall L, et al. (1998). False-positive HIV-1 test results
in a low -risk screening setting of voluntary blood donation. Journal of the
American Medical Association 280:1080-1083.
|


 Home
Home What the Perth Group has argued
What the Perth Group has argued
 Papadopulos redox theory of cellular function papers
Papadopulos redox theory of cellular function papers Papers and letters published in scientific journals
Papers and letters published in scientific journals Monograph on mother-to-child transmission
Monograph on mother-to-child transmission
 Papers published in Continuum magazine
Papers published in Continuum magazine
 Papers published in the popular press
Papers published in the popular press
 Papers/letters rejected by the scientific press
Papers/letters rejected by the scientific press
 Presentations
Presentations
 Interviews
Interviews
 Selected email correspondence
Selected email correspondence
 A virus like no other
A virus like no other
 Oxidation, Montagnier and the Perth Group
Oxidation, Montagnier and the Perth Group
 Montagnier Nobel Prize 2008
Montagnier Nobel Prize 2008
 The Parenzee Case
The Parenzee Case
 The House of Numbers
The House of Numbers
 Latest files
Latest files
 National Libary of Australia Intervew 1993
National Libary of Australia Intervew 1993
 Others
Others
 Africa/South Africa
Africa/South Africa
 Questions and answers
Questions and answers
 Response to the NIH "Evidence" that HIV causes AIDS
Response to the NIH "Evidence" that HIV causes AIDS
 Translations of the Perth Group papers
Translations of the Perth Group papers
 BMJ Online Debate
BMJ Online Debate

 Contact Us
Contact Us
 About the Perth Group
About the Perth Group
 Perth Group at Virusmyth
Perth Group at Virusmyth
 The Perth Group on YouTube
The Perth Group on YouTube